Featured Member Archive
Seham Ebrahim
Member
I am an Assistant Professor in the Department of Molecular Physiology and Biological Physics, University of Virginia School of Medicine. I was born in Karachi and grew up in Dubai I did postdoctoral work at NIH with Drs Bechara Kechar and Roberto Weigert after my PHD (Biological Sciences, Queen Mary University of London). My laboratory works on the mechanobiology of the cellular response to environmental changes. Our focus is on cytoskeletal network dynamics networks during channel mechano sensing in intestinal epithelia during inflammation, cancer, and aging.
See “www.med.virginia.edu/ebrahim-lab” for more.

Publication
In vivo, the cytoskeletal components actin and non-muscle myosin II (NMIIA) employ myriad distinct architectures, dynamics, and interactions to conduct specialized functions. These architectures cannot be fully recapitulated and studied in in vitro. Intravital microscopy imaging allowed the study of the 4D cellular physiology of the cytoskeleton in native tissues.
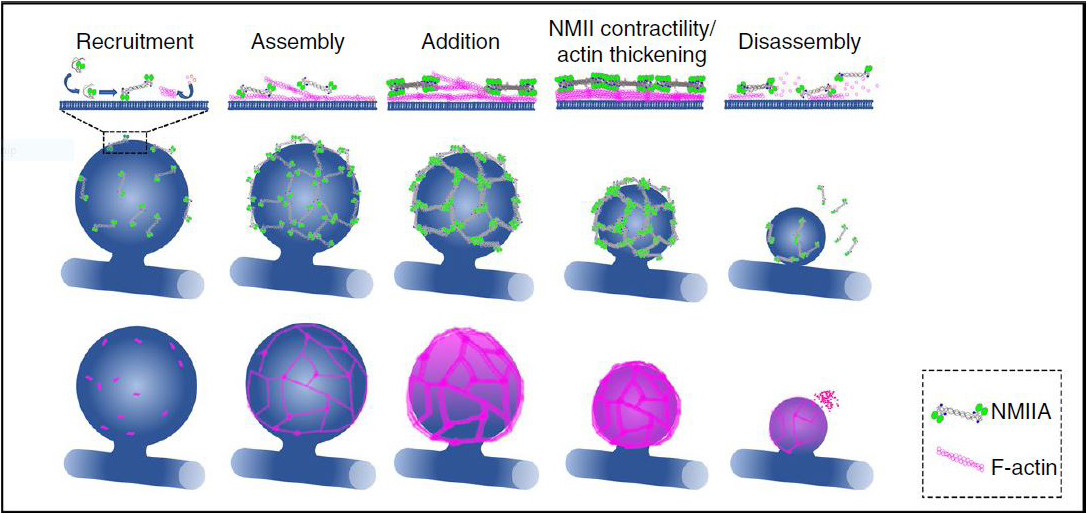
The study used four-dimensional spinning-disc confocal microscopy with image deconvolution to acquire macromolecular-scale detail of dynamic actomyosin networks in exocrine glands of live mice. We addressed how actin and NMIIA organize into previously undescribed polyhedral-like lattices around large membrane-bound secretory vesicles and generate the forces required to complete exocytosis. We show using photobleaching and pharmacological perturbations in vivo that actomyosin contractility and actin polymerization together push on the underlying vesicle membrane to complete exocytosis. The Figure shows a model for lattice assembly and dynamics (see1 for details).
- Ebrahim, S. et al. Dynamic polyhedral actomyosin lattices remodel micron-scale curved membranes during exocytosis in live mice. Nat Cell Biol 21, 933-939 (2019).
Humayun Sharif
Member
Humayun Sharif trained as a structural biologist with Elena Conti at the Max Planck Institute of Biochemistry (Munich, Germany). He has MS (GIST, South Korea) and BS from (MAJU, Pakistan) in Bioinformatics. He is currently an instructor at Harvard Medical School/Boston Children’s Hospital with Hao Wu, following postdoctoral stints with Wu and Michael Eck at the same institution. He studies host-pathogen interaction and the innate immune response. He has a special interest in using structure-driven approaches for the elucidation of programmed cell-death pathways.

Publication
Inflammasomes are cytoplasmic supramolecular complexes that form in response to either microbial invasions or endogenous cell damage. Upon activation, inflammasomes initiate explosive programmed cell death (Pyroptosis). NLRP3 is one of the most studied inflammasome, but the molecular details of NLRP3 inflammasome activation remain elusive.
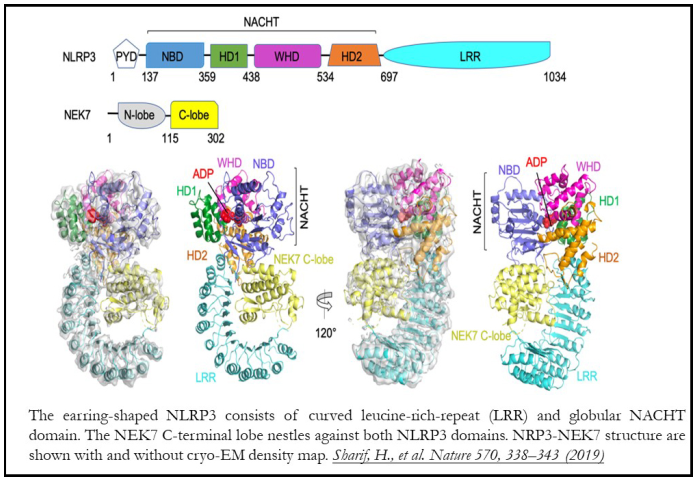
The study presented the cryo-electron microscopy (cryo-EM) structure of NLRP3 with NEK7 kinase and adenosine-dinucleotide (ADP) at 3.8 Å resolution. Structure determination by cryo-EM visualizes biomolecular assemblies in their native hydrated state at near atomic resolution due to the mechanical stability, low electron dose and image capture capabilities of modern detectors and electron microscopes. Associated advances in single-particle image processing algorithms allow assessment of structural heterogeneity over a large size range difficult to achieve by other methods. The size, heterogeneity, and flexibility of the NLRP3-NEK7 complex make cryo-EM the method of choice for its study.
Since NLRP3 is in an activated conformation in the complex, a homologous activated inflammasome structure was used for de novo modeling. This bipartite interaction of NEK7 with NLRP3-LRR in a disk-like assembly was revealed to be critical for activation. Almost all NLRP3 mutations lie in NACHT domain and surround the ADP-binding pocket. The structure rationalizes how these mutations compromise interdomain interactions of NACHT or disrupt the ADP-binding pocket required for NLRP3 activation. These insights were validated with subsequent biochemical and cellular assays. They will be valuable in guiding the design of inhibitors for treatment of CAPS and other inflammatory diseases.
Mohsin Khan
Member
I am an Assistant Professor at Center for Metabolic Disease Research, Lewis Katz School of Medicine, Temple University. I did my PhD from Center for Excellence in Molecular Biology (CEMB), University of the Punjab, Lahore, Pakistan in Molecular Biology on the “role of stem cells for the repair of aging heart”. Postdoctoral work was conducted in Dr. Mark Sussman’s lab, San Diego State University, studying the role of β-adrenergic signaling in cardiac progenitor cells and in Dr. Raj Kishore’s lab, Northwestern University, on the role of exosomes in cardiac regeneration.
please visit www.mkhanlab.com for more information.

Publication
The neonatal cardiac tissue is a proliferative organ capable of regenerating lost myocardium after injury. This regenerative window is short lived, as the cardiomyocytes differentiate terminally and exit the cell cycle. The adult heart has limited ability for cardiac regeneration with estimates for less than 1% cellular turnover throughout lifetime. The fundamental question is whether the adult cardiac tissue can be transformed to a development state enhancing cardiac repair and regeneration. We have previously shown that delivery of embryonic factors to the heart via exosomes enhances cardiac repair following myocardial infarction. We have identified miR-294 as one of the critical embryonic factors with potential beneficial effects on cardiac repair. miR-294 is an embryonic cell cycle microRNA making up 70% of the entire microRNA content in the embryonic stem cells and regulates core functions such as cell cycle, pluripotency, self-renewal and proliferation. Nevertheless, the effect of miR-294 specifically on the heart and on cardiomyocyte cell cycle has never been previously studied.

We have now documented for the first time a role for miR-294 as a driver of pro-reparative changes in the heart. These include increased cardiomyocyte cell cycle reentry and cell survival, induction of angiogenesis, restriction of infarct size and enhanced developmental molecular signaling in the heart. miR-294 represses Wee-1 kinase that dephosphorylates CDK1 releasing CDK1/Cyclin B1 complex from Wee-1 mediated inactivation, thereby promoting cell cycle reentry in cardiomyocytes (Figure). Our delivery strategy employed a Tet-regulated AAV9-based approach for the miRNA induction in the heart for 14 days following myocardial injury. The duration goes beyond any study for miRNA delivery to the heart to date. Follow-up of mice for up to 8 weeks after miR-294 administration showed enhanced cardiac function together with increased cardiomyocyte replenishment after myocardial infarction injury.
